Research Article
Early Screening and Prevention Strategies for Cervical Cancer Based on Genetic Markers 
2 Liuliqiao Outpatient Department, Jingnan Medical Area of PLA General Hospital, Beijing, 100161, Beijing, China
 Author
Author  Correspondence author
Correspondence author
International Journal of Molecular Medical Science, 2024, Vol. 14, No. 5
Received: 16 Jul., 2024 Accepted: 24 Aug., 2024 Published: 06 Sep., 2024
Cervical cancer remains a significant public health issue, especially in low- and middle-income countries. While traditional screening methods such as Pap smears and HPV DNA testing have reduced the incidence and mortality of cervical cancer, they are limited in sensitivity, specificity, and the ability to differentiate between transient and persistent high-risk infections. This study reviews the current status of cervical cancer screening and prevention, with a focus on the role of genetic markers in enhancing early screening accuracy and guiding personalized prevention strategies. The paper also addresses challenges associated with implementing these advanced methods, including cost, accessibility, ethical considerations, and the need for healthcare system adaptation. Recent advancements in genetic marker-based screening, such as next-generation sequencing (NGS), DNA methylation panels, and emerging biomarkers like microRNAs (miRNAs), have opened up new avenues for early detection and more precise risk stratification. Embracing these advancements can enable healthcare systems to enhance early detection and prevention of cervical cancer, ultimately reducing its global burden.
1 Introduction
Cervical cancer remains one of the most prevalent gynecological cancers globally, particularly impacting women in low- and middle-income countries (Zhang et al., 2022). The primary cause of this disease is persistent infection with high-risk types of human papillomavirus (HPV), which can lead to precancerous lesions and, if left untreated, progress to invasive cancer (Li et al., 2020). Despite advancements in screening techniques, including the Papanicolaou (Pap) smear and HPV DNA testing, cervical cancer continues to be a significant public health challenge, especially in areas with limited healthcare resources and insufficient screening coverage (Lu et al., 2020). Early detection and prevention are vital for reducing the morbidity and mortality associated with this disease, highlighting the urgent need for improved screening strategies that can more effectively identify high-risk individuals (Lee et al., 2020).
Recent advances in molecular biology have underscored the significance of genetic and epigenetic markers in the early detection and prevention of cervical cancer. High-risk HPV infection triggers a series of genetic and epigenetic changes in host cells, playing a key role in cervical carcinogenesis (Malik et al., 2023). Genetic markers, such as DNA methylation patterns, gene mutations, and alterations in microRNA (miRNA) expression, have demonstrated greater specificity and sensitivity in identifying high-grade precancerous lesions compared to traditional cytology-based methods (Jeannot et al., 2021). For example, DNA methylation markers have emerged as a promising strategy for triaging HPV-positive women, helping to distinguish between transient infections and those that could progress to cervical intraepithelial neoplasia (CIN) (Wittenborn et al., 2020; Tu et al., 2022). These markers provide deeper insights into the molecular changes linked to cervical cancer development, opening new pathways for early screening, risk stratification, and targeted prevention strategies (Gradissimo and Burk, 2017).
This study aims to examine the current landscape of early screening and prevention strategies for cervical cancer, with a particular emphasis on the role of genetic markers. By synthesizing the latest advancements in genetic marker screening methods, this research highlights their potential to enhance early detection and improve prevention efforts, ultimately reducing the global burden of cervical cancer. Understanding the significance of these markers in identifying high-risk individuals will support the development of more effective and targeted screening programs, especially in resource-limited settings where traditional methods may be less accessible or feasible. The study will also address the challenges and opportunities associated with implementing genetic marker screening across various healthcare environments, offering guidance for future research and informing public health strategies focused on the early detection and prevention of cervical cancer.
2 Current Screening Methods for Cervical Cancer
2.1 Pap smear testing
The Papanicolaou (Pap) smear test, introduced in the 1940s, has become a cornerstone of cervical cancer screening, significantly contributing to the reduction of cervical cancer incidence and mortality, especially in high-income countries (Comparetto and Borruto, 2021). This test involves collecting exfoliated cells from the cervix and examining them under a microscope to identify abnormalities that may indicate precancerous changes or early-stage cancer (Figure 1). The Pap smear is particularly effective in detecting cervical intraepithelial neoplasia (CIN), enabling early intervention before lesions progress to invasive cancer (AieshaKhatun and Gudi, 2021). Routine Pap smear screening has been linked to a substantial decline in cervical cancer rates in countries with established screening programs, as it facilitates the detection and treatment of lesions at a stage when they are most amenable to intervention (Wardle et al., 2015). In many high-income countries, organized screening programs recommend regular Pap testing every three to five years for women aged 21 to 65, depending on age and risk factors (Bruni et al., 2022). This interval reflects the natural progression of cervical cancer, which typically takes several years to develop from an initial HPV infection to invasive disease.
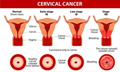 Figure 1 Staging of cervical cancer (Adapted from Mustafa et al., 2020) Image caption: The figure illustrates the progression of lesions from early to advanced stages; Early-stage cervical cancer is typically confined to the cervical region, whereas advanced cancer may have invaded the surrounding tissues; The staging in the figure clearly delineates the characteristics of different stages; for example, early stages are mainly limited to the epithelial cells of the cervix, while advanced stages can involve deeper tissue structures; This staging is crucial for the diagnosis and treatment of cervical cancer, helping physicians assess the severity of the cancer and develop appropriate treatment plans; The figure also underscores that the progression of cervical cancer is a gradual process, highlighting the importance of early screening and diagnosis (Adapted from Mustafa et al., 2020) |
Despite its widespread use and success, the Pap smear has several limitations. One major concern is its variable sensitivity, which can range from 50% to 75%, influenced by factors like sample quality, the presence of adequate cellular material, and the expertise of the cytologist interpreting the results (Arbyn et al., 2012). False-negative results can occur, potentially missing cases of high-grade lesions, particularly when cellular abnormalities are subtle or if sampling errors happen during specimen collection. Additionally, the Pap smear is primarily a cytological test that detects morphological changes in cervical cells but does not provide insights into the underlying molecular alterations, such as genetic or epigenetic changes that drive cervical carcinogenesis (Liang et al., 2021). This limitation can be especially problematic in the early stages of cervical cancer development, when cellular changes may not yet be apparent. Consequently, there is a growing recognition of the need for more sensitive and specific screening tools, such as genetic marker-based methods, which could complement or even replace cytology-based screening to improve early detection and reduce the burden of cervical cancer.
2.2 HPV DNA testing
Human papillomavirus (HPV) DNA testing has become a crucial part of cervical cancer screening due to the established role of high-risk HPV types in its development (Sabeena et al., 2020). This test detects the presence of DNA from high-risk HPV strains in cervical cells, helping to identify women at increased risk for developing cervical intraepithelial neoplasia (CIN) and cervical cancer (Zhang et al., 2022). HPV DNA testing has shown superior sensitivity compared to cytology-based methods, with sensitivity rates exceeding 90% for detecting CIN2+ lesions (Thomsen et al., 2021). This high sensitivity makes HPV DNA testing an invaluable tool for identifying at-risk women, facilitating earlier intervention and treatment. Additionally, HPV DNA testing can serve as a primary screening method or as a triage option following an abnormal cytology result, thereby enhancing the overall effectiveness of the screening strategy.
One major advantage of HPV DNA testing is its ability to be conducted using self-collected samples, which has been shown to increase screening uptake, especially among populations with limited access to healthcare services or those less likely to participate in regular screening programs. Self-sampling methods, such as vaginal swabs, have proven to be highly acceptable and effective, offering a way to expand screening coverage in low-resource settings and among women hesitant to undergo clinician-collected sampling (Leeuwen et al., 2018). Additionally, the high negative predictive value of HPV DNA testing allows for longer screening intervals, easing the burden on healthcare systems and reducing the frequency of invasive procedures for women with negative results. However, while HPV DNA testing significantly enhances the detection of high-risk cases, its relatively low specificity can lead to the identification of transient HPV infections that may never progress to cancer (Gaisa et al., 2021). This limitation highlights the need for complementary triage methods, such as genetic or epigenetic markers, to better identify women at the highest risk of developing cervical cancer.
2.3 Limitations of traditional screening methods
Despite the widespread use of Pap smears and HPV DNA testing in cervical cancer screening programs, both methods have significant limitations that can affect their overall effectiveness. The Pap smear, while crucial in reducing the incidence and mortality of cervical cancer, has variable sensitivity, ranging from 50% to 75%. This variability depends on factors such as sample collection, slide preparation, and the expertise of the observer in cytological interpretation. As a result, false-negative results can occur, causing precancerous or cancerous lesions to be missed and delaying diagnosis and treatment. Additionally, the subjective nature of cytology interpretation means the Pap smear may overlook subtle cellular changes, particularly in the early stages of carcinogenesis, when molecular alterations have yet to manifest as visible abnormalities (Comparetto and Borruto, 2021). Consequently, while the Pap smear is effective for detecting established lesions, it may be less reliable for early detection of cervical cancer at the molecular level.
HPV DNA testing, in contrast, provides higher sensitivity for detecting high-risk HPV infections linked to cervical cancer. However, this increased sensitivity comes with a trade-off: reduced specificity. HPV DNA testing can identify transient HPV infections, which are common and often resolve on their own without causing significant disease (Zhang et al., 2022). As a result, a positive HPV test does not necessarily indicate an immediate risk of developing cervical cancer, leading to a high rate of false positives. This can result in unnecessary follow-up procedures, such as colposcopies and biopsies, which increase healthcare costs and may cause both physical and psychological distress for patients. Moreover, while HPV testing is effective at detecting high-risk HPV types, it does not provide information about the subsequent genetic and epigenetic changes in host cells that drive cancer progression. Therefore, while traditional screening methods like Pap smears and HPV DNA testing are vital components of current cervical cancer prevention strategies, they have inherent limitations that can impede early detection and precise risk stratification (Ntanasis-Stathopoulos et al., 2020). This highlights the need for more advanced screening approaches that incorporate genetic markers to better identify women at high risk for cervical cancer, ultimately improving early detection and reducing the incidence of overtreatment.
3 Genetic Markers Linked to Cervical Cancer
3.1 HPV-related genetic mutations
Persistent infection with high-risk human papillomavirus (HPV) types, particularly HPV16 and HPV18, is recognized as the primary cause of cervical cancer (Table 1). The integration of the viral genome into the host cell DNA disrupts regulatory genes and leads to the overexpression of viral oncoproteins E6 and E7, resulting in the inactivation of tumor suppressor proteins like p53 and retinoblastoma protein (pRb) (Keating et al., 2001). This process initiates a cascade of genetic changes within the host cell, contributing to the development of precancerous lesions and invasive cervical cancer. Recent research has identified several host cell genes that are upregulated in association with HPV infection, acting as potential surrogate markers for HPV-related epithelial lesions. For example, genes involved in cell cycle regulation, such as TP53, CDKN2A, and MYC, show mutations or altered expression in HPV-positive cervical cancer cases, helping to identify individuals at high risk for cancer progression (Salama et al., 2022). These genetic alterations offer insights into the mechanisms of HPV-induced carcinogenesis and present promising targets for early screening and risk stratification.
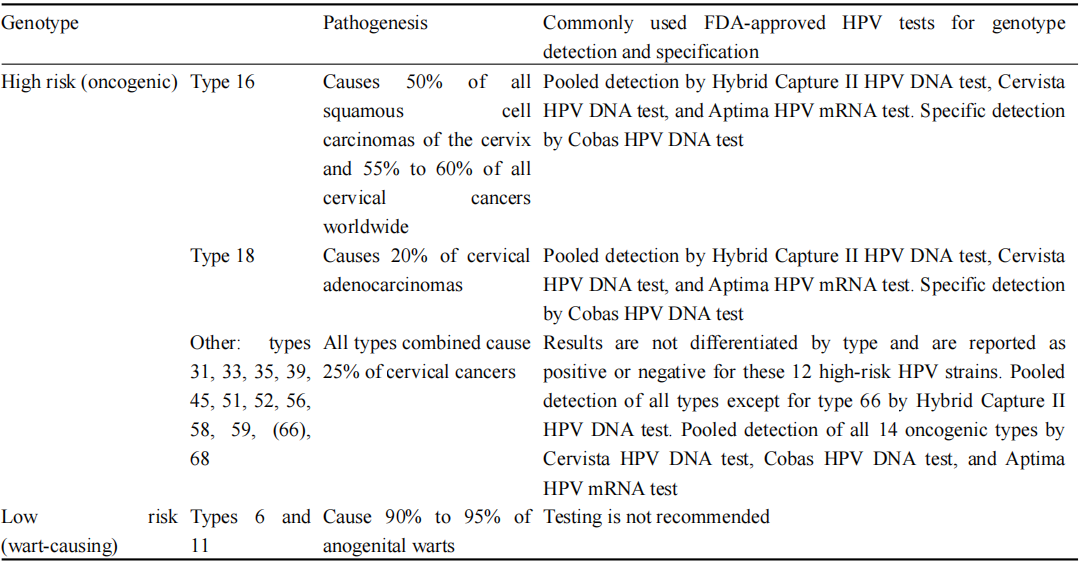 Table 1 Clinically important HPV genotypes (Adopted from Rerucha et al., 2018) |
3.2 Epigenetic changes
In addition to direct genetic mutations, epigenetic modifications like DNA methylation play a crucial role in the development of cervical cancer. HPV infection can induce epigenetic changes in both the viral and host genomes, resulting in the silencing of tumor suppressor genes and the activation of oncogenic pathways. Methylation of specific gene promoters, including those of tumor suppressor genes such as CDH1, MGMT, and DAPK, has been linked to the development of cervical cancer (Reis et al., 2020). Studies indicate that detecting DNA methylation markers in cervical cells can be an effective triage tool for identifying women with high-grade cervical intraepithelial neoplasia (CIN2+) among HPV-positive individuals. Additionally, methylation of the HPV genome itself, particularly in the L1 and L2 regions, has been associated with the persistence of HPV infection and progression to cervical cancer (Zhang et al., 2022). These epigenetic markers provide a non-invasive approach for early detection, potentially enhancing the specificity of cervical cancer screening and reducing unnecessary interventions.
3.3 Biomarkers beyond HPV
While HPV-related genetic and epigenetic alterations are central to cervical carcinogenesis, other molecular markers beyond HPV have also been identified as potential contributors to disease progression. Recent research has focused on microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), which play crucial roles in regulating gene expression and are often dysregulated in cancer. Specific miRNAs, such as miR-21, miR-34a, and miR-143-3p, have shown altered expression in cervical cancer and precancerous lesions, correlating with disease severity (Wittenborn et al., 2020). These miRNAs can serve as both diagnostic and prognostic markers, adding valuable information beyond traditional HPV testing. Additionally, protein biomarkers like p16INK4a, Ki-67, and cyclin E—associated with cell proliferation and cycle regulation-have been proposed as surrogate markers for HPV-related oncogenesis and are being explored for their potential in cervical cancer screening and diagnosis. These biomarkers could enhance the accuracy of screening by helping to distinguish between benign HPV infections and lesions at higher risk of progressing to invasive cancer, ultimately improving early detection and patient management.
4 Advances in Genetic Marker-based Screening
4.1 Next-generation sequencing (NGS)
Next-Generation Sequencing (NGS) has transformed the field of genetic screening by providing high-throughput, comprehensive analysis of genetic alterations linked to cervical cancer (Zhong, 2024). NGS enables the simultaneous sequencing of multiple genes, including those associated with HPV integration and host cell mutations, offering detailed insights into the genetic landscape of cervical lesions (Lee et al., 2020). By detecting somatic mutations, single nucleotide variants (SNVs), and copy number variations (CNVs), NGS can uncover specific genetic signatures related to the progression of precancerous lesions to invasive cervical cancer. For example, mutations in genes like PIK3CA, TP53, and PTEN have been identified in cervical cancer patients through NGS, providing potential markers for early detection and personalized risk assessment (Espinosa et al., 2013). Moreover, NGS-based liquid biopsy approaches, which analyze circulating tumor DNA (ctDNA) in the blood, are being investigated for their potential as a non-invasive screening method that can monitor genetic changes in real time, supporting early detection and treatment monitoring.
4.2 DNA Methylation panels
DNA methylation, an epigenetic modification that regulates gene expression, has emerged as a promising marker for the early detection of cervical cancer. The methylation of specific gene promoters, both in the host and the HPV genome, has been linked to the silencing of tumor suppressor genes and the progression of cervical intraepithelial neoplasia (CIN) to invasive cancer. Methylation panels, which assess the methylation status of multiple genes, have been developed as triage tools to identify high-grade lesions among women with HPV-positive results. For example, a panel that includes methylation markers such as ANKRD18CP, C13ORF18, and EPB41L3 has shown high sensitivity and specificity in distinguishing CIN2+ lesions from lower-grade abnormalities (Li et al., 2021). Using DNA methylation panels alongside HPV testing has the potential to enhance the accuracy of cervical cancer screening, thereby reducing unnecessary colposcopies and biopsies by providing a more precise risk assessment. Additionally, these panels can be applied to self-collected samples, facilitating broader population-based screening, particularly in low-resource settings where access to healthcare facilities is limited.
4.3 Machine learning in screening
The integration of machine learning and artificial intelligence (AI) into cervical cancer screening has the potential to significantly enhance the interpretation of genetic and epigenetic data, leading to more accurate and efficient screening strategies (Hou et al., 2022). Machine learning algorithms can analyze complex datasets, including genomic, methylation, and imaging data, to identify patterns linked to cervical cancer risk. For instance, ensemble learning techniques have been used to predict cervical cancer risk by combining genetic markers with clinical features, thereby improving the robustness and accuracy of risk stratification models. By incorporating gene-assistance modules and data correction mechanisms, these machine learning models can tackle the challenges of variability and ambiguity in cervical cancer screening, providing personalized risk assessments and supporting clinical decision-making. Additionally, AI-based image analysis of cervical cytology and histology samples has shown promise in reducing observer variability and increasing sensitivity in detecting precancerous lesions. The combination of machine learning with genetic marker-based screening could revolutionize cervical cancer screening programs, enabling more precise and early identification of high-risk individuals while optimizing resource allocation.
5 Genetic Markers for Cervical Cancer Prevention
5.1 Prophylactic vaccination and genetic markers
Prophylactic vaccination against high-risk HPV types, particularly HPV16 and HPV18, has proven to be a highly effective primary prevention strategy for cervical cancer. Vaccines like Gardasil and Cervarix have shown significant reductions in the incidence of HPV infections and related cervical lesions. Recent research suggests that genetic markers could enhance the effectiveness of HPV vaccination programs by identifying individuals who are more likely to benefit from vaccination and those who may require additional interventions. For example, studies indicate that variations in the human leukocyte antigen (HLA) system may influence individual susceptibility to HPV infection and the response to vaccination (Espinosa et al., 2013). Understanding these genetic markers can help optimize vaccination strategies by targeting high-risk populations and monitoring vaccine efficacy more effectively. Additionally, genetic markers associated with immune response may inform the development of next-generation vaccines with broader coverage, enhancing their protective effects against various oncogenic HPV strains (Zhang et al., 2022).
5.2 Personalized prevention based on genetic risk
The identification of genetic markers linked to an increased risk of cervical cancer paves the way for personalized prevention strategies (Chen, 2024). Individuals with specific genetic predispositions, such as alterations in genes involved in DNA repair, cell cycle regulation, and immune response, may be more likely to develop cervical cancer following HPV infection. Personalized prevention programs can incorporate genetic risk assessments to identify these high-risk individuals, allowing for tailored interventions like more frequent screenings, earlier initiation of screening, or the use of additional diagnostic tools such as DNA methylation panels. Furthermore, personalized strategies can inform decisions regarding HPV vaccination for older individuals or those who may have already been exposed to HPV, maximizing the vaccine's preventive potential by considering genetic susceptibility (Cohen et al., 2020). By integrating genetic risk factors with traditional screening and vaccination programs, personalized prevention has the potential to more effectively and efficiently reduce the incidence of cervical cancer.
5.3 Genetic testing in primary prevention
Genetic testing has become an increasingly vital tool in primary prevention efforts for cervical cancer. Advances in genetic testing technologies, such as next-generation sequencing (NGS) and polymerase chain reaction (PCR)-based assays, enable the identification of high-risk genetic markers, including mutations in host genes and epigenetic modifications linked to an elevated risk of cervical cancer. This testing can pinpoint women at higher risk who may benefit from intensified screening protocols, even before any clinical symptoms or cytological abnormalities arise. For instance, women with specific genetic alterations, such as those in the TP53 or CDKN2A genes, may require more rigorous surveillance due to their increased likelihood of developing high-grade cervical lesions. Additionally, testing for DNA methylation markers has shown promise as a triage tool for HPV-positive women, offering a more precise risk assessment that can guide decisions on further diagnostic procedures and interventions. By integrating genetic testing into primary prevention strategies, healthcare providers can take a more proactive approach to cervical cancer prevention, ultimately reducing the burden of this disease through early intervention and targeted management.
6 Challenges in Implementing Genetic Marker-Based Screening and Prevention
6.1 Cost and accessibility issues
One of the main challenges in implementing genetic marker-based screening for cervical cancer is the associated cost. Advanced technologies like next-generation sequencing (NGS) and DNA methylation testing can be expensive, limiting their accessibility, particularly in low- and middle-income countries (LMICs), where cervical cancer rates are often highest (Zhang et al., 2022). The costs of equipment, reagents, and the necessity for skilled personnel to perform and interpret these tests can lead to high per-patient expenses, making widespread adoption difficult. In resource-limited settings, the infrastructure for standard screening methods like Pap smears may already be inadequate, let alone the advanced technologies needed for genetic testing. Additionally, the cost-effectiveness of genetic screening is still under evaluation; implementing these advanced techniques may not always result in lower overall healthcare costs due to the potential need for follow-up procedures for positive or unclear results. Consequently, finding a balance between the benefits of early detection and the economic constraints of healthcare systems presents a significant challenge.
6.2 Ethical concerns in genetic screening
The integration of genetic marker-based screening raises several ethical concerns, particularly related to privacy, consent, and the risk of genetic discrimination. Genetic testing can disclose sensitive information about an individual’s predisposition not only to cervical cancer but also to other hereditary conditions, prompting questions about how this information will be used and who has access to it (Wardle et al., 2015). Informed consent is critical, as individuals need to fully understand the implications of genetic testing, including the psychological effects of discovering an increased cancer risk and the associated anxiety. Moreover, there is a risk of genetic discrimination by employers or insurance companies, raising concerns about the confidentiality and security of genetic information. To address these issues, ethical guidelines and robust legal frameworks are necessary to protect individuals' rights and ensure responsible conduct in genetic screening. Tackling these ethical challenges is essential for the widespread acceptance and implementation of genetic marker-based screening programs.
6.3 Healthcare system adaptation
Implementing genetic marker-based screening and prevention strategies necessitates significant adaptations within healthcare systems. This involves developing appropriate clinical guidelines, training healthcare providers, and ensuring the necessary infrastructure and technology are in place (Lu et al., 2020). Healthcare providers must be trained not only in administering and interpreting genetic tests but also in counseling patients about the results and their implications for clinical management. Furthermore, integrating genetic testing into existing screening programs requires standardized protocols for follow-up care, including risk stratification and management for individuals identified as high risk. Additionally, healthcare systems need to establish reliable data management systems to handle complex genetic information securely and accurately. In many regions, especially low- and middle-income countries (LMICs), the lack of infrastructure and trained personnel presents a significant barrier to adopting genetic marker-based screening. Therefore, healthcare systems must invest in technology, workforce training, and sustainable strategies to make genetic screening accessible and effective on a larger scale.
7 Future Directions in Cervical Cancer Screening and Prevention
7.1 Integration of genetic and traditional methods
Future advancements in cervical cancer screening are likely to involve integrating genetic marker-based screening with traditional methods like Pap smears and HPV DNA testing. This combined approach aims to enhance both the sensitivity and specificity of screening programs, improving early detection rates while minimizing false positives. For example, co-testing with HPV DNA and Pap smears has already proven more effective in identifying high-grade lesions than either method alone. Incorporating genetic markers, such as DNA methylation patterns and gene mutations, into this screening framework could further refine risk stratification. Women who test positive for high-risk HPV but show no cytological abnormalities might undergo additional genetic marker testing to evaluate their risk of progression to cervical cancer, thus reducing unnecessary colposcopy referrals and overtreatment (Zhang et al., 2022). This integration marks a shift toward a more personalized screening approach, allowing for more targeted interventions and enhancing the overall efficiency of cervical cancer prevention programs.
7.2 Innovative biomarker research
Ongoing research into novel biomarkers offers exciting potential for the future of cervical cancer screening. In addition to DNA methylation and HPV-related genetic mutations, emerging biomarkers such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and protein-based markers are being explored for their ability to enhance early detection and risk assessment. For instance, microRNAs like miR-21, miR-143-3p, and miR-34a have been found to be dysregulated in cervical cancer and show promise as non-invasive biomarkers for early diagnosis. Similarly, protein markers such as p16INK4a and Ki-67, which are linked to cellular proliferation and oncogenic transformation, are being investigated as complementary tools to current screening methods. These markers could help differentiate between transient HPV infections and lesions with a higher risk of progression. Research into these and other biomarkers is likely to lead to the development of multi-modal screening panels that combine various types of molecular markers, providing a more comprehensive risk profile. This could significantly improve the accuracy of early screening and facilitate timely interventions.
7.3 AI and predictive modeling
Artificial Intelligence (AI) and predictive modeling are set to play a crucial role in the future of cervical cancer screening and prevention. Machine learning algorithms can analyze complex datasets-encompassing genetic, epigenetic, and clinical information-to uncover patterns that may indicate early disease or heightened risk. AI has already demonstrated its potential in improving the interpretation of Pap smear images, detecting subtle abnormalities that might be overlooked by human observers. Moreover, predictive models that integrate genetic markers, clinical histories, and other risk factors can offer personalized risk assessments, tailoring the frequency and type of screening for each individual. For instance, AI-driven algorithms can help stratify HPV-positive women according to their genetic risk of progressing to high-grade cervical lesions, enabling more customized follow-up and management (Keating et al., 2001). The incorporation of AI and machine learning into cervical cancer screening is anticipated to enhance the precision and efficiency of early detection strategies, reduce healthcare costs, and ultimately improve patient outcomes by ensuring that high-risk individuals receive timely and appropriate care.
8 Concluding Remarks
The landscape of cervical cancer screening and prevention is rapidly evolving with the incorporation of genetic markers. While traditional methods like Pap smears and HPV DNA testing have significantly lowered cervical cancer rates, they still face challenges related to specificity and distinguishing between transient and persistent high-risk infections. Advances in genetic marker-based screening-such as next-generation sequencing (NGS), DNA methylation panels, and emerging biomarkers like microRNAs (miRNAs)-present new opportunities for more accurate early detection and risk stratification. Additionally, integrating genetic testing into primary prevention strategies, including personalized risk assessments and targeted HPV vaccination programs, could significantly enhance the effectiveness of cervical cancer prevention efforts. However, challenges such as cost, accessibility, ethical considerations, and the need for healthcare system adaptations must be addressed to ensure equitable access to these advanced screening options. The use of artificial intelligence (AI) and predictive modeling further enhances the potential of genetic markers, providing personalized screening strategies that can optimize outcomes and help reduce the global burden of cervical cancer.
The future of cervical cancer screening and prevention hinges on developing and implementing a multi-modal approach that combines traditional screening methods with advanced genetic marker-based technologies. This integration could significantly enhance early detection of high-grade lesions while reducing overtreatment by providing more precise and individualized risk assessments. Ongoing research into novel biomarkers, such as microRNAs (miRNAs) and protein markers, will further improve the ability to accurately identify at-risk individuals when incorporated into screening panels.As healthcare systems adapt to these advancements, it’s essential to address barriers related to cost and accessibility, especially in low-resource settings where the burden of cervical cancer is highest. Ethical considerations, including informed consent and genetic privacy, must also be prioritized to ensure that the benefits of genetic screening are realized responsibly and equitably.
Moreover, incorporating AI and machine learning into screening programs will enable the development of predictive models that deliver personalized screening and prevention strategies, ultimately improving patient outcomes and reducing cervical cancer incidence and mortality worldwide. By embracing these future directions, we can fully realize the promise of genetic marker-based screening and prevention strategies, paving the way for a new era in cervical cancer control.
Acknowledgments
We thank the anonymous reviewers for their insightful comments and suggestions for the manuscript.
Funding
This work was not supported by any individual or organization.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
AieshaKhatun N., and Gudi S.N., 2021, A comparative study of visual inspection with acetic acid and papsmear in screening cervical intraepithelial neoplasia, Sch Int J ObstetGynec, 4(6): 241-249.
Arbyn M., Ronco G., Anttila A., Meijer C., Poljak M., Ogilvie G., Koliopoulos G., Nauclér P., Sankaranarayanan R., and Peto J., 2012, Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer, Vaccine, 30(5): F88-F99.
https://doi.org/10.1016/j.vaccine.2012.06.095
Bruni, L., Serrano, B., Roura, E., Alemany, L., Cowan, M., Herrero, R., Poljak P., Murillo R., Broutet N., Riley L., and de Sanjose S., 2022, Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis, The Lancet Global Health, 10(8): e1115-e1127.
https://doi.org/10.1016/S2214-109X(22)00241-8
Chen S.Y., 2024, Optimizing drug therapy using genomic information: a pathway to personalized medicine, International Journal of Molecular Medical Science, 14(1): 61-68.
http://dx.doi.org/10.5376/ijmms.2024.14.0009
Cohen A.C., Roane B.M., and Leath III C.A., 2020, Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy, Drugs, 80(3): 217-227.
https://doi.org/10.1007/s40265-019-01249-z
Comparetto C., and Borruto F., 2021, Genetic screening of cervical cancer, OBM Genetics, 5(3): 1-37.
https://doi.org/10.21926/obm.genet.2103132
Espinosa A., Alfaro A., Román-Basaure E., Guardado-Estrada M., Palma Í., Serralde C., Medina I., Juárez E., Bermúdez M., Marquez E., Borges-Ibáñez M., Muñoz-Cortez S., Alcántara-Vázquez A., Alonso P., Curiel-Valdez J., Kofman S., Villegas N., and Berumen J., 2013, Mitosis is a source of potential markers for screening and survival and therapeutic targets in cervical cancer, PLoS ONE, 8(2): e55975.
https://doi.org/10.1371/journal.pone.0055975
Gaisa M.M., Sigel K.M., Deshmukh A.A., Lenskaya V., Chan C.A., Silvera R., Winters J., and Liu Y., 2021, Comparing anal cancer screening algorithms using cytology and human papillomavirus DNA testing in 3 high-risk populations, The Journal of infectious diseases, 224(5): 881-888.
https://doi.org/10.1093/infdis/jiaa801
Gradissimo A., and Burk R., 2017, Molecular tests potentially improving HPV screening and genotyping for cervical cancer prevention, Expert Review of Molecular Diagnostics, 17: 379-391.
https://doi.org/10.1080/14737159.2017.1293525
Hou X., Shen G., Zhou L., Li Y., Wang T., and Ma X., 2022, Artificial intelligence in cervical cancer screening and diagnosis, Frontiers in Oncology, 12: 851367.
https://doi.org/10.3389/fonc.2022.851367
Jeannot E., Latouche A., Bonneau C., Calméjane M., Beaufort C., Ruigrok-Ritstier K., Bataillon G., Chérif L., Dupain C., Lecerf C., Popovic M., Rochefordière A., Lécuru F., Fourchotte V., Jordanova E., Leyen H., Tran-Perennou C., Legrier M., Dureau S., Raizonville L., Roufai D., Tourneau C., Bièche I., Rouzier R., Berns E., Kamal M., and Scholl S., 2021, Circulating HPV DNA as a marker for early detection of relapse in patients with cervical cancer, Clinical Cancer Research, 27(21): 5869-5877.
https://doi.org/10.1158/1078-0432.CCR-21-0625
Keating J., Ince T., and Crum C., 2001, Surrogate biomarkers of HPV infection in cervical neoplasia screening and diagnosis, Advances in Anatomic Pathology, 8: 83-92.
https://doi.org/10.1097/00125480-200103000-00004
Lee S., Chae D., Lee S., Lim Y., An J., Chae C., Kim B., Bhak J., Bolser D., and Cho D., 2020, Efficient mutation screening for cervical cancers from circulating tumor DNA in blood, BMC Cancer, 20: 1-10.
https://doi.org/10.1186/s12885-020-07161-0
Leeuwen R., Oštrbenk A., Poljak M., Zee A., Schuuring E., and Wisman G., 2018, DNA methylation markers as a triage test for identification of cervical lesions in a high risk human papillomavirus positive screening cohort, International Journal of Cancer, 144: 746-754.
https://doi.org/10.1002/ijc.31897
Li N., Hu Y., Zhang X., Liu Y., He Y., Zee A., Schuuring E., and Wisman G., 2020, DNA methylation markers as triage test for the early identification of cervical lesions in a Chinese population, International Journal of Cancer, 148: 1768-1777.
https://doi.org/10.1002/ijc.33430
Liang L.A., Einzmann T., Franzen A., Schwarzer K., Schauberger G., Schriefer D., Radde K., Zeissig S., Ikenberg H., Meijer C., Kirkpatrick C., Kölbl H., Blettner M., and Klug S.J., 2021, Cervical cancer screening: comparison of conventional Pap smear test, liquid-based cytology, and human papillomavirus testing as stand-alone or cotesting strategies, Cancer Epidemiology, Biomarkers & Prevention, 30(3): 474-484.
https://doi.org/10.1158/1055-9965.EPI-20-1003
Lu J., Song E., Ghoneim A., and Alrashoud M., 2020, Machine learning for assisting cervical cancer diagnosis: an ensemble approach, Future Gener. Comput. Syst., 106: 199-205.
https://doi.org/10.1016/j.future.2019.12.033
Malik S., Sah R., Muhammad K., and Waheed Y., 2023, Tracking HPV infection, associated cancer development, and recent treatment efforts-a comprehensive review, Vaccines, 11(1): 102.
https://doi.org/10.3390/vaccines11010102
Mustafa W.A., Halim A., Jamlos M.A., and Idrus S.Z.S., 2020, A review: Pap smear analysis based on image processing approach, Journal of Physics: Conference Series, 1529( 2): 022080.
https://doi.org/10.1088/1742-6596/1529/2/022080
Ntanasis-Stathopoulos I., Kyriazoglou A., Liontos M., Dimopoulos M.A., and Gavriatopoulou M., 2020, Current trends in the management and prevention of human papillomavirus (HPV) infection, J. Buon, 25(3): 1281-1285.
https://doi.org/10.2478/fco-2019-0014
Reis R.S.D., Santos J.A.D., Abreu P.M.D., Dettogni R.S., Santos E.D.V.W.D., Stur E., Agostini L., Anders Q., Alves L., Valle I., Lima M., Souza E., Podestá J., Zeidler S., Cordeiro-Silva M., and Louro I.D., 2020, Hypermethylation status of DAPK, MGMT and RUNX3 in HPV negative oral and oropharyngeal squamous cell carcinoma, Genetics and Molecular Biology, 43(3): e20190334.
https://doi.org/10.1590/1678-4685-gmb-2019-0334
Rerucha C.M., Caro R.J., and Wheeler V.L., 2018, Cervical cancer screening, American Family Physician, 97(7): 441-448.
Sabeena S., Kuriakose S., Damodaran B., Ravishankar N., and Arunkumar G., 2020, Human papillomavirus (HPV) DNA detection in uterine cervix cancer after radiation indicating recurrence: a systematic review and meta-analysis, Journal of Gynecologic Oncology, 31(2): e20.
https://doi.org/10.3802/jgo.2020.31.e20
Salama A.M., Momeni-Boroujeni A., Vanderbilt C., Ladanyi M., and Soslow R., 2022, Molecular landscape of vulvovaginal squamous cell carcinoma: new insights into molecular mechanisms of HPV-associated and HPV-independent squamous cell carcinoma, Modern Pathology, 35(2): 274-282.
https://doi.org/10.1038/s41379-021-00942-3
Thomsen L.T., Kjær S.K., Munk C., Ørnskov D., and Waldstrøm M., 2021, Benefits and potential harms of human papillomavirus (HPV)‐based cervical cancer screening: a real‐world comparison of HPV testing versus cytology, Acta Obstetricia et Gynecologica Scandinavica, 100(3): 394-402.
https://doi.org/10.1111/aogs.14121
Tu J., Chen S., Wu S., Wu T., Fan R., and Kuang Z., 2022, Tumor DNA methylation profiles enable diagnosis, prognosis prediction, and screening for cervical cancer, International Journal of General Medicine, 15: 5809-5821.
https://doi.org/10.2147/IJGM.S352373
Wardle J., Robb K., Vernon S., and Waller J., 2015, Screening for prevention and early diagnosis of cancer, The American psychologist, 70(2): 119-133.
https://doi.org/10.1037/a0037357
Wittenborn J., Weikert L., Hangarter B., Stickeler E., and Maurer J., 2020, The use of miRNA in the early detection of cervical intraepithelial neoplasia, Carcinogenesis, 43: 89-89.
https://doi.org/10.1093/carcin/bgaa046
Zhang L., Tan W., Yang H., Zhang S., and Dai Y., 2022, Detection of host cell gene/HPV DNA methylation markers: a promising triage approach for cervical cancer, Frontiers in Oncology, 12: 831949.
https://doi.org/10.3389/fonc.2022.831949
Zhong J.L., 2024, Epigenetic biomarkers in patients with hypertensive heart disease, International Journal of Molecular Medical Science, 14(2): 132-143.
http://dx.doi.org/10.5376/ijmms.2024.14.0016
(1).png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Shanshan Li
. Mingzi Huang
. Chunyan Ji
Related articles
. Cervical cancer
. Genetic markers
. Early screening
. DNA methylation
. Next-generation sequencing (NGS)
. Artificial intelligence (AI)
Tools
. Post a comment